Empowering
Healthcare
Consumers
Atomo Diagnostics Limited is a global leader in the user-centred design of innovative, accurate and reliable rapid diagnostics.
Atomo Diagnostics delivers lab-level performance at the point-of-care.
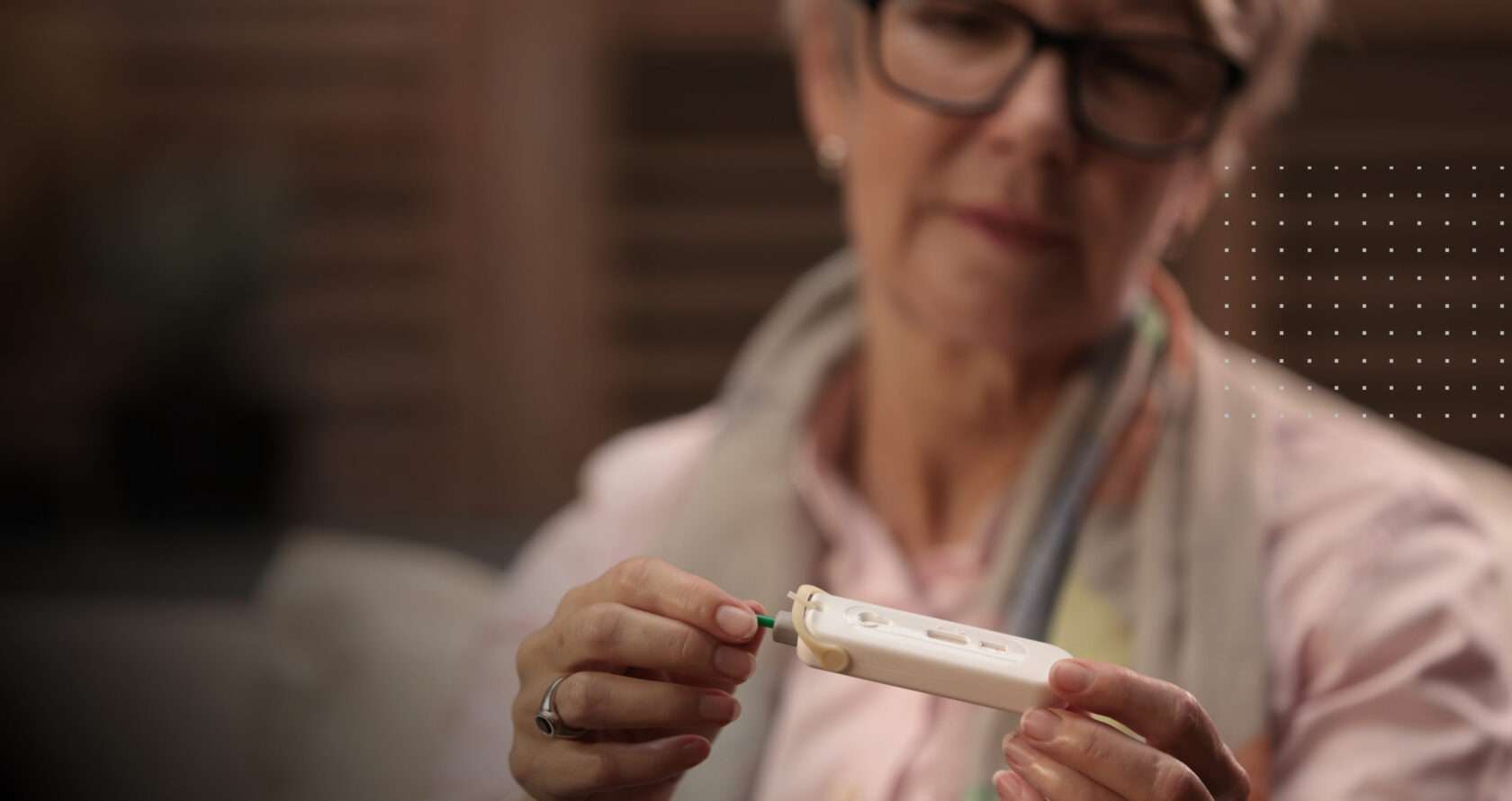
Atomo Diagnostics Technology Centers Around User Needs
Atomo Diagnostics is the first company in the world to bring human-centred engineering to the heart of diagnostics design. We focus on how tests operate in the hands of users to improve ease of use and reduce error rates. This leads to better user experience, more accurate results and better health care outcomes.

Together We Can Transform Diagnostics
Partner with Atomo Diagnostics for an efficient, low-risk pathway from product development to commercialisation of your best-in-class rapid diagnostic test. Today’s users are demanding more. They expect more choices in rapid tests that can reliably be done at home, giving them more control and keeping them more informed. Now is the time to innovate.
Our unique, proprietary technologies improve rapid diagnostics testing by elevating ease-of-use and performance.

Unmatched Usability
User-centred design for professional use & self-test applications.

Proven Performance
Award-winning device platforms that are proven to mitigate user errors.

Expert Development
Knowledge & experience to accelerate new custom devices.

Tests Sold

User Preference





